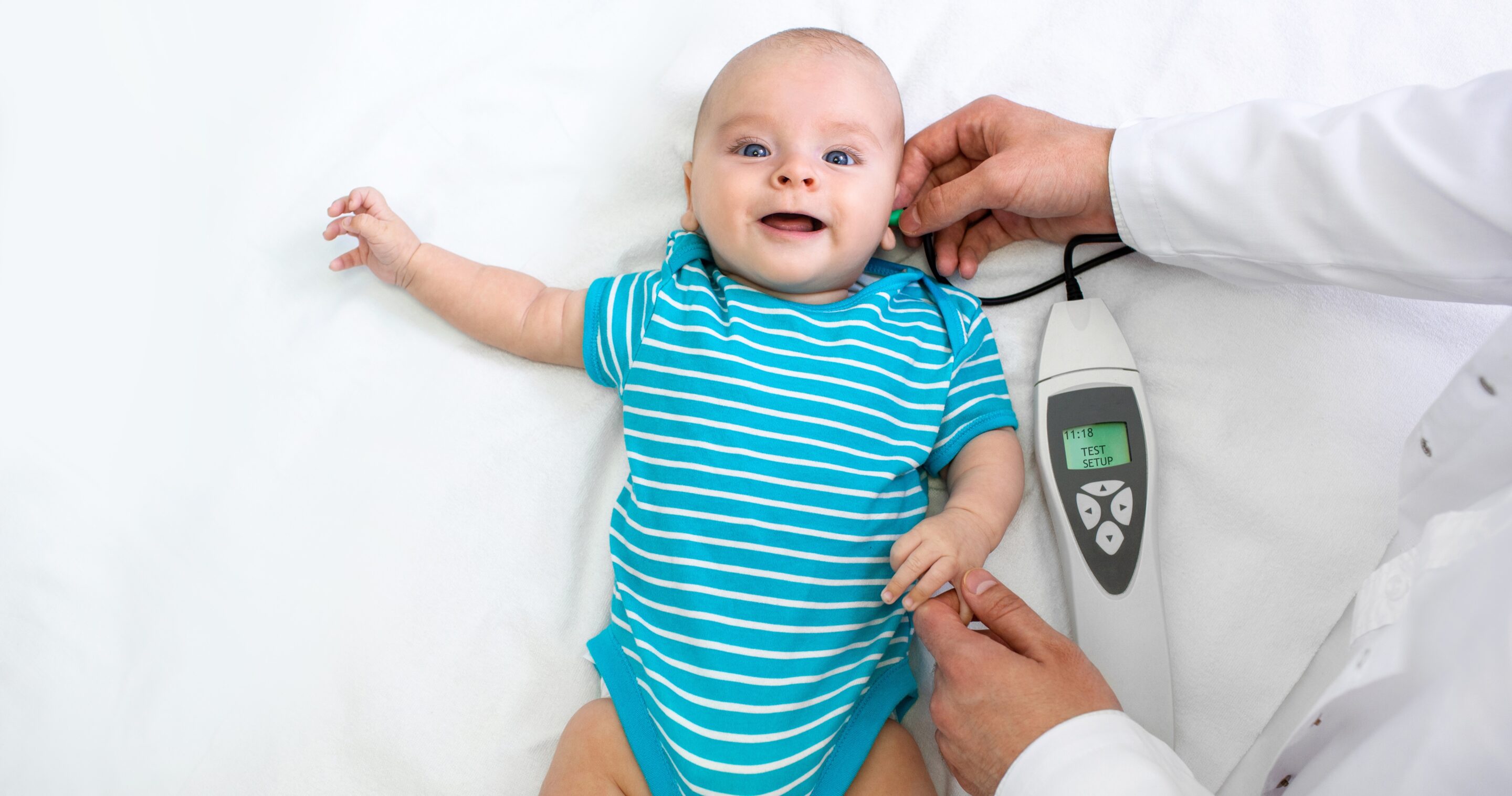
The gene therapies developed by Sensorion could restore the natural hearing of young patients under the age of 3. Instead of hearing 22 frequencies at best, as is the case today with a cochlear implant, they would be able to access a much wider spectrum of frequencies (e.g., the sound of the voice of a close relative, better spatial orientation, listening to music and learning to speak faster).
Currently, 1.7 children out of 1,000 are born deaf in the United States (Source: Center for Disease Control CDC). In half of all cases, this deafness is caused by a genetic mutation, with around a hundred genes affecting hearing.
With this first trial, Sensorion has decided to target OTOF, the gene responsible for otoferlin, a protein essential for the proper functioning of hearing. Otoferlin deficiency only affects around 1,100 births each year in Europe and the United States. We need to convince the medical community, parents and regulatory authorities,” says Sensorion CEO Nawal Ouzren. The experience we will gain with this first treatment will benefit the second, which targets a much larger population.”
Another therapy based on the GJB2 gene is currently under development. This gene is responsible for half of all genetic birth defects, i.e. at least 20,000 babies a year in Europe and the United States. The biotech is expected to apply for clinical trial approval in 2025.
In the meantime, priority is given to the gene therapy targeting OTOF, SENS-501, which is injected once. Improvements are expected to appear as early as one month after injection, and to continue for three to four months. A first dosage will be tested on three infants aged 6 to 31 months born deaf and otoferlin-deficient, then a second, stronger dosage on three others, and finally, depending on the results, a third will be determined and administered to the rest of the cohort, i.e. six very young patients in France, Italy, Germany and Great Britain. In France, they will be monitored at Hôpital Necker Enfants Malades AP-HP.
“This innovation is a massive breakthrough in medical practice. Our gene therapies could restore natural hearing to young children born deaf,” says Sensorion CEO Nawal Ouzren.
Close collaboration with the Institut Pasteur
Created in 2009 in Montpellier, in the south of France, Sensorion will be collaborating from 2019 onwards with the Institut de l’Audition, a world-class center of excellence that is part of the Institut Pasteur. In Prof. Christine Petit’s laboratory, they are developing viral vectors capable of restoring the normal functioning of target cells where the protein should be expressed.
Recently, the Children’s Hospital of Philadelphia (CHOP) enabled an 11-year-old suffering from this otoferlin deficiency to regain his hearing, also thanks to gene therapy. At this age, he will probably never be able to speak properly, since this learning takes place before the age of 5. Sensorion is therefore one of the first biotech companies in the world to start a trial targeting babies.
“For this world first, we are relying on cochlear implant specialists in ten centers in Europe,” explains Nawal Ouzren. When they detect a genetic birth defect, these specialists systematically genotype the child and offer to enroll him or her in Sensorion’s clinical study, with meticulous follow-up.
Initial results from the clinical study evaluating the efficacy of SENS-501 are expected in the second half of this year.