Until two years ago, Marc fell several times a day. He barely left his house. The 60-year-old from Bordeaux was diagnosed with Parkinson’s disease almost 30 years ago. In 2021, he underwent surgery at the Centre Hospitalier Universitaire Vaudois (CHUV) in Lausanne. He is the first Parkinson’s patient to receive a new neuroprosthesis, consisting of a field of electrodes placed against the area of the spinal cord that controls walking along with an electrical impulse generator implanted under the skin of the abdomen. After several weeks of physiotherapy to familiarize himself with the device and to personalize the neuroprosthesis algorithm according to his movement, Marc noticed considerable progress, and his neuromotor problems disappeared. He has regained almost regular use of his legs, with no loss of balance or “freezing”, a phenomenon common in the advanced stages of the disease where the feet remain glued to the ground. Today, Marc can walk five kilometers without stopping.
This feat marks the culmination of nearly 14 years of work by around fifty neuroscientists, all co-authors of an article published in Nature Medicine on 6 November 2023. They give new hope to the approximately 8.5 million people with Parkinson’s disease, according to 2019 figures from the World Health Organisation. It is estimated that 90% of them have problems walking (Inserm).
A European project
The device was conceived and designed by the teams of Grégoire Courtine, a neuroscientist at the Ecole polytechnique fédérale de Lausanne (EPFL), and Jocelyne Bloch, a neurosurgeon at the Centre Hospitalier Universitaire Vaudois, in collaboration with Erwan Bézard, a neuroscientist and Inserm research director at the Institut des Maladies Neurodégénératives (University of Bordeaux/CNRS).
It all started in 2009 with a bid for a European project to enable paraplegic patients to walk again. The neuroprosthesis was first tested on rats (2014), primates (2016) and paraplegic human subjects (2018). The operation was a success each time and was the subject of publications in Nature. The neuroscientists then decided to extend their solution to Parkinson’s patients, who can walk, but with difficulty.
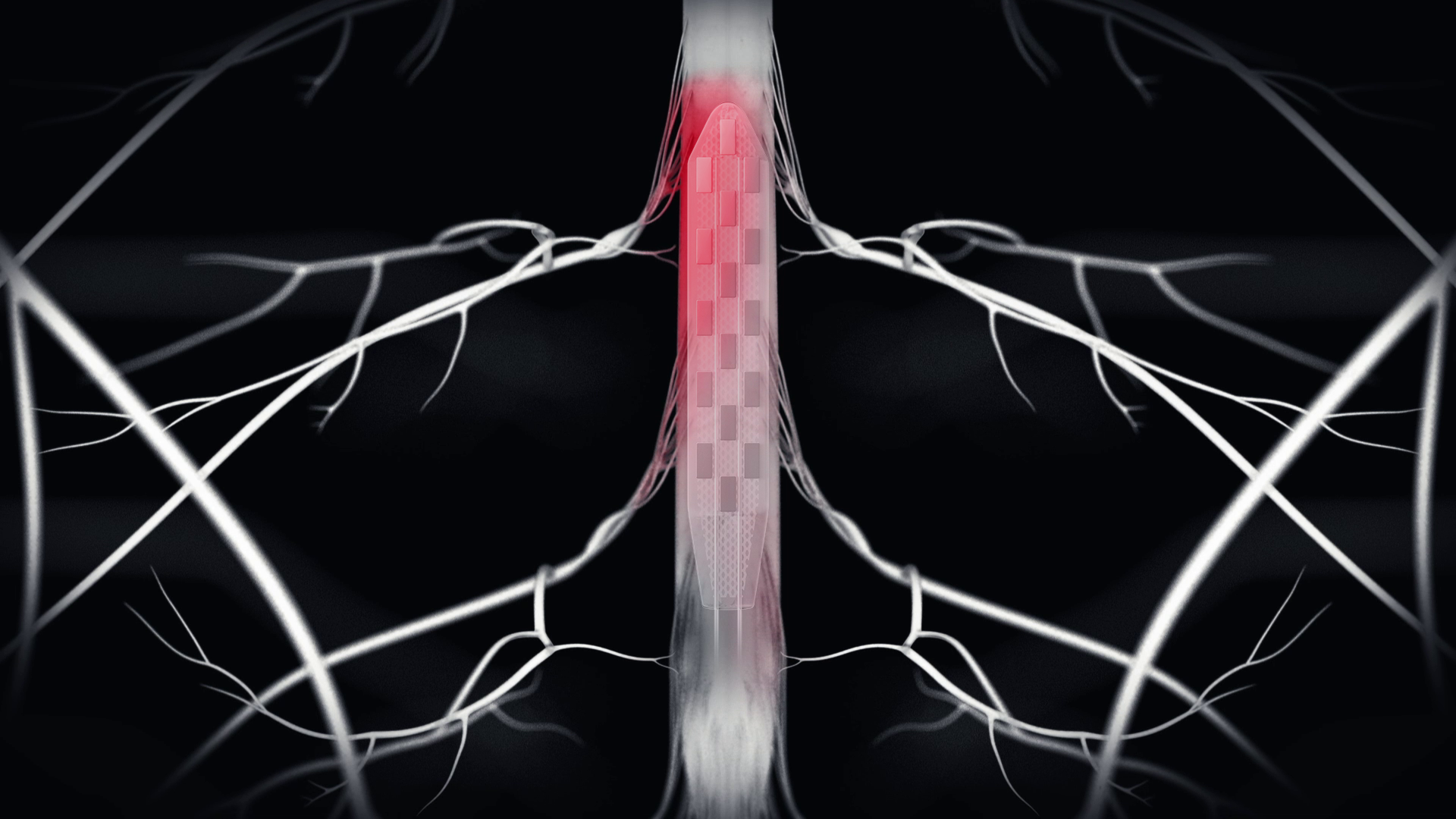
A strip equipped with 16 electrodes is placed on the lumbosacral region, i.e. between the envelope of the spinal cord (the dura mater) and the bone. A cable runs to the stimulator, which is around 5 cm in diameter and located in the abdomen. It contains the stimulation algorithm, which gives it instructions on how to stimulate the subject in a controlled way, spatially and temporally, according to the subject’s natural gait.
“The spinal cord is not stimulated all at once, but the motor neurons of each muscle involved in walking are stimulated sequentially. Both sides of the spinal cord are stimulated in an orderly, adapted manner to match the patient’s functioning as closely as possible. (…) The intention to walk is triggered by accelerometers placed on the shin or in connected shoes, which in turn trigger the algorithm, via Bluetooth, which in turn triggers the stimulator, which provides the electricity to stimulate the electrodes according to a predetermined spatio-temporal plan“, explains Erwan Bézard, Inserm research director at the Institute of Neurodegenerative Diseases (University of Bordeaux/CNRS).
A clinical trial involving six Parkinson’s patients will begin in 2024, over 18 months, funded by the Michael J. Fox Foundation. The aim will be to demonstrate once again that this global equipment restores their ability to walk smoothly. If successful, it will be followed by a three-year multicenter study involving twenty patients. The finished product could be available to the general public within four to five years.