Over 179 million people in Europe suffer from at least one form of neurological disorder, often caused by the accumulation of toxic proteins in the neurons that spread from cell to cell via as yet poorly understood processes.
Microglia are the brain’s first line of defense against invading pathogens. They are activated in the event of inflammation. The question now is to assess the positive or negative impact of such inflammation on the spread of neurodegenerative diseases. Does it help the patient survive, or does it contribute to neuronal death, thus making the disease worse? For 20 years now, Chiara Zurzolo and her team of researchers have been studying the mechanisms that lead to neuronal death.
They began by targeting the prion function of bovine spongiform encephalopathy (BSE), also known as mad cow disease, bearing in mind that the protein replicates itself in a way that makes it toxic.
“The prion protein is capable of replicating itself in a disordered manner, becoming toxic in the process,” said the Director of the Brain Connectivity and Neurodegenerative Diseases Research Axis at the Institut Pasteur. “It can pass from one cell to another, from the intestine to the brain, and is therefore considered infectious. Huntington’s, Parkinson’s and Alzheimer’s diseases have also been shown to contain toxic aggregates called amyloids, that are similar to toxic prion proteins.”
Successive discoveries made have enabled scientists to show that the disease gradually spreads to different parts of the brain, as a result of different protein aggregates. “The question that needs to be answered is how do these aggregates move from one part of the brain to another causing neuronal death, which may or may not lead to peripheral dementia-like symptoms,” the Director of Research added.
The first paper was published in 2009 in Nature Cell Biology. In it, the research team presented their findings on the role of TNTs in the propagation of the prion protein and, subsequently, other amyloids such as aSyn (in Parkinson’s disease) and Tau protein (in Alzheimer’s disease).
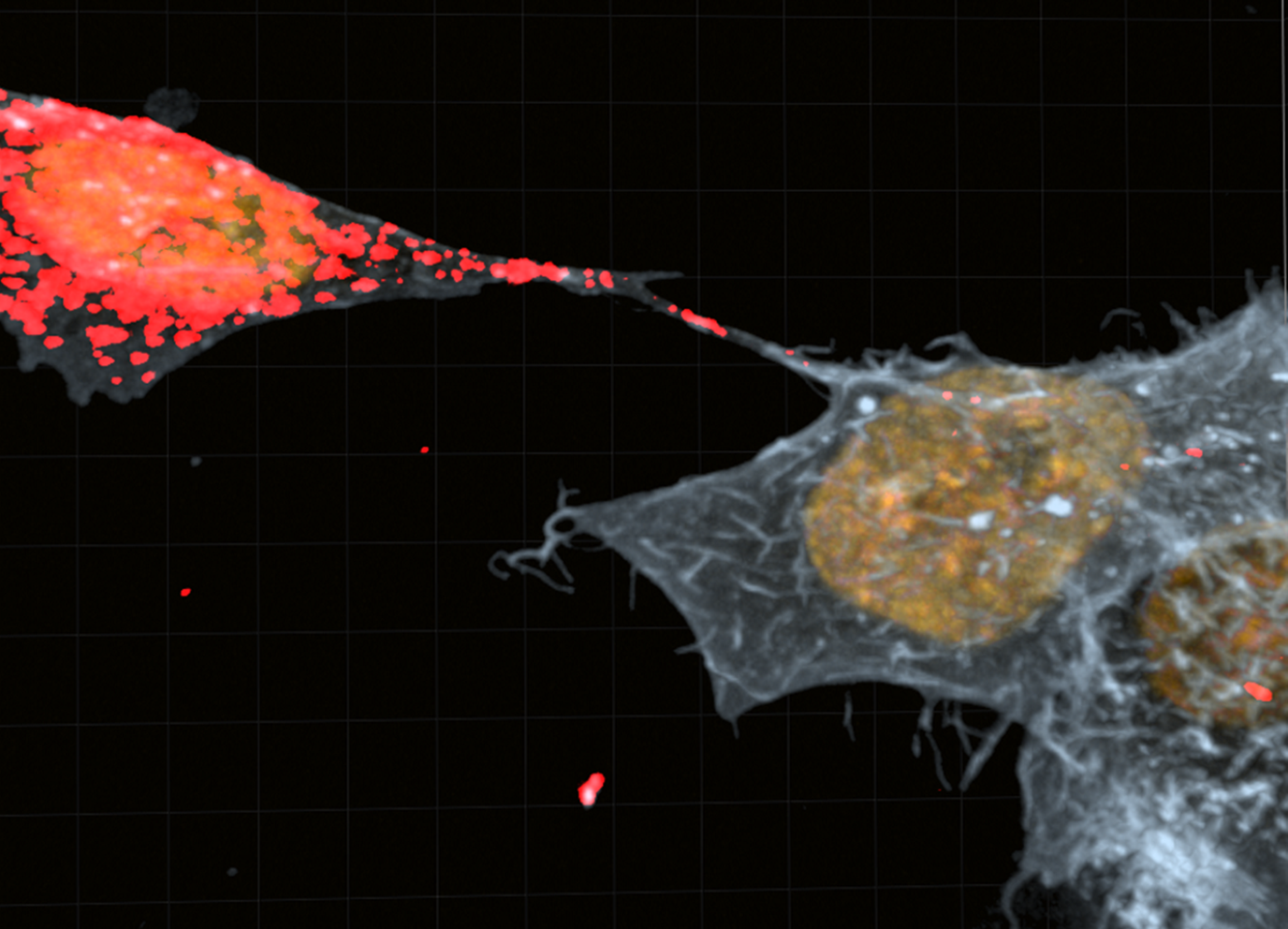
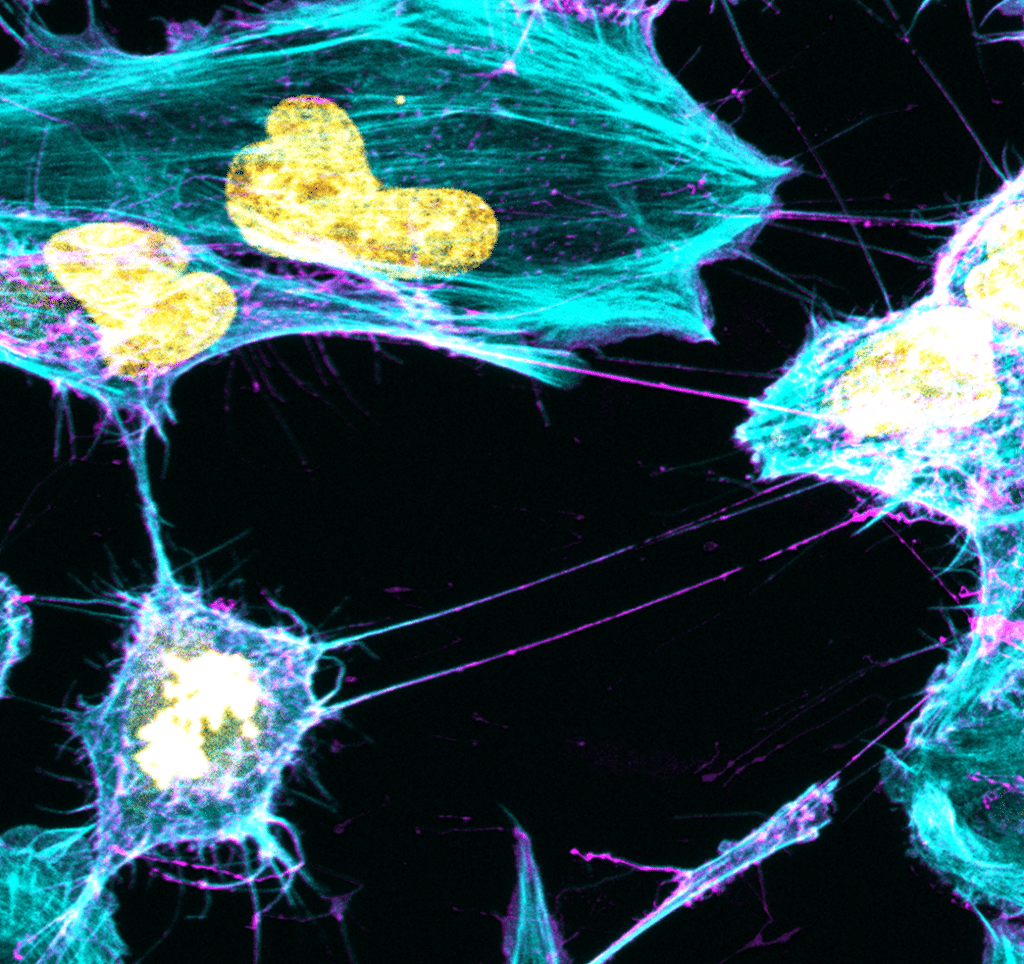
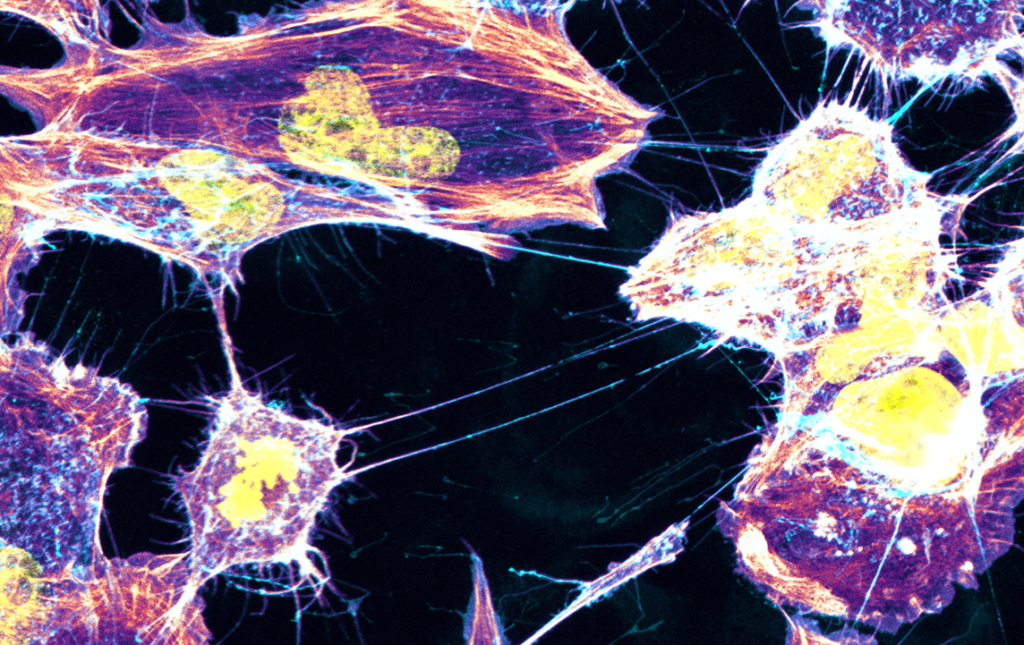
The Membrane Traffic and Pathogenesis Unit then demonstrated in August 2016 that structures called TNTs (tunneling nanotubes) facilitate the transfer of toxic proteins between neurons, before hypothesizing that TNTs play a major role in the spread of aggregates throughout the brain.
Following the publication of half a dozen articles over recent years, including one in 2017 on the role of astrocytes, the most recent publication in May 2023 sheds light on the role of microglia. According to this work, they act as a pathway for the spread of alpha-synuclein, a toxic protein responsible for Parkinson’s disease. “Neurons loaded with alpha-synuclein aggregates made effective use of TNTs to transfer them to microglia, probably with the aim of reducing their volume in neurons. This is the first time that the existence of functional connections between neurons and glial cells has been demonstrated,”, said Ranabir Chakraborty, a PhD student in the unit and co-author of the study.
“This discovery could open up new avenues of treatment to see if healthy microglia can be used to help diseased neurons, but we need to continue our research to understand in what inflammatory state microglia are in the brain,” Chiara Zurzolo, Director of the Brain Connectivity and Neurodegenerative Diseases Research Axis at the Institut Pasteur.
The researchers also showed that glial cells are able to transfer mitochondria to unhealthy neurons, again thanks to TNTs.
There’s still a long way to go, and there are many hypotheses. “For example, we could assume that in the early stages of the disease, microglia try to save neurons. Then, when the inflammatory state changes, they become aggressive,” said Chiara Zurzolo. “Instead of saving the neuron, they eat it, so we would need to intervene at a very early stage.” These results open up new fields of research into communication between neurons and microglia, and potentially new therapeutic targets regarding the role of inflammation, not only in Parkinson’s disease but also in all other degenerative diseases. “If we understand how TNTs spread, we can try to block them and use immune system cells to stop the disease, as for cancers with immunotherapies, provided we find the right targets for neurodegenerative diseases,” Chiara Zurzolo said.