At its launch in 2017, Fizimed based its operations on a simple observation: 1 woman in 10, in France, suffers from bladder weakness. “This subject although taboo is, nevertheless, quite normal,” said Emeline Hahn, CEO. In the beginning, physiotherapists informed her of the need for better support for women in their homes, in addition and subsequent to perineal re-education sessions, with professionals, including midwives. There is a wide spectrum of patients concerned. It ranges from post-partum, to women suffering from bladder weakness, problems during sexual relations, organ descent or also relaxation of the muscles of the perineum during menopause.
“Fizimed normalises these taboo subjects linked to female healthcare, by providing simple solutions to an extremely common albeit little known problem, perineal relaxation,” Emeline Hahn, CEO.
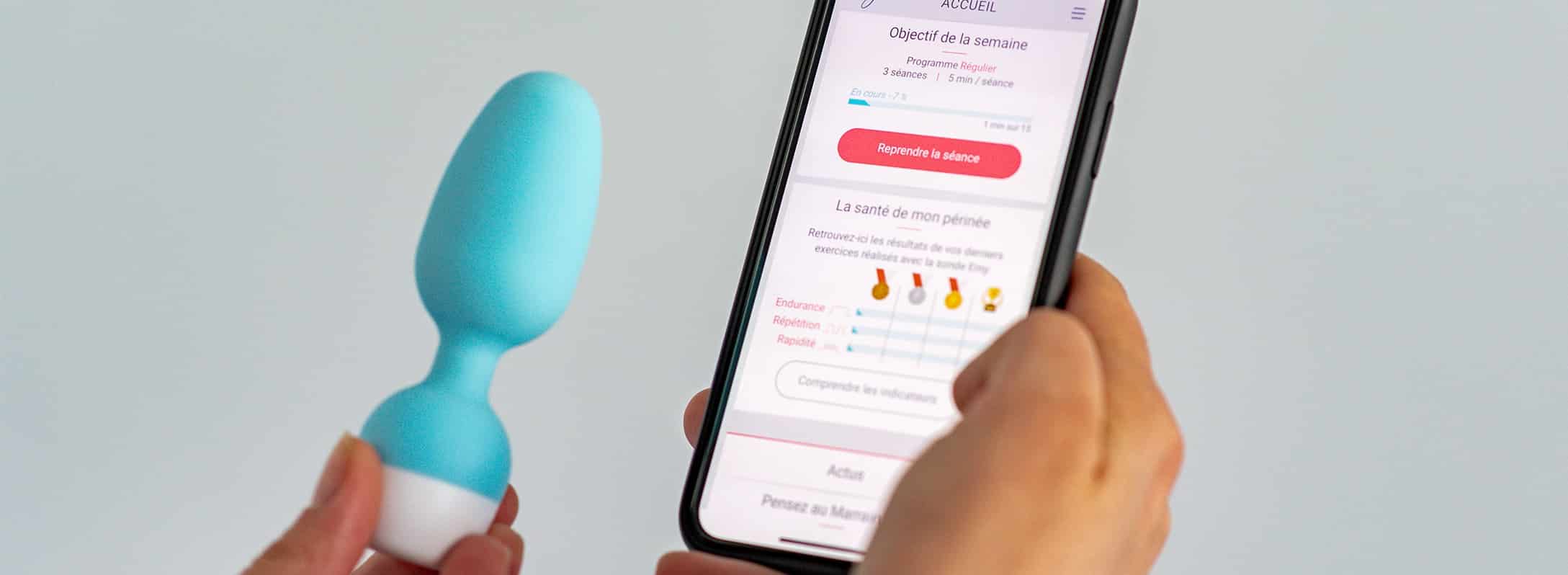
The probe, made from medical-grade materials, operates using Bluetooth. Connected to an application, it works in a way that is fun. The patient has a choice of 20 mini-games based on medical protocols, contraction and relaxation exercises. She can therefore follow her long-term progress via a range of indicators. The aim is for her to use it for 5 to 20 minutes several times a week, for the period of time judged necessary. The device can, of course, be used again, if it becomes necessary.
A clinical study carried out over two years, in university hospitals in Strasbourg, in the east of France, demonstrated the effectiveness of the medical device. 98% of women noted a clear improvement in their symptoms.
The product is currently sold in pharmacies and online, on specialist sites, in Europe and Asia. It costs €199.
Fizimed is now trying to penetrate the U.S. market. The start-up already has a subsidiary in Chicago. In addition to offering its device to healthcare establishments and gynaecologists, it hopes to continue with its social initiative and feels it has an education mission in this area.
Fizimed is supported by the French Healthcare NEXT program, which has already enabled it to better understand the U.S. market and its regulations and to plan meetings with key health actors in the U.S. healthcare ecosystem.