Calcific aortic stenosis (CAS) is the most common valvular heart disease in Europe, with a mortality rate of over 50% at 1 year following invasive treatment. In this degenerative disease, the aortic valve (positioned between the left ventricle and the aorta) calcifies, becomes rigid and can no longer open properly, leading to heart failure or sudden death. This problem affects between 1.7% and 12.4% of people over the age of 75. As the population ages, these figures are set to rise.
The conventional standard of care today is to replace the dysfunctional valve either by open-chest surgery or, in 90% of cases, by a percutaneous procedure known as TAVI (“Transcatheter Aortic Valve Implantation”). But these two invasive procedures are cumbersome; 16% of patients classified as having severe symptomatic stenosis cannot undergo them. Age, level of frailty, or stage of the disease can disqualify many from the procedure.
For such patients, a research team from French academic laboratories Inserm and APHP have tested a new approach called “non-invasive ultrasound therapy” (or NIUT). The technology was then developed by Cardiawave, a spin-off from a collaboration between the Hôpital Européen Georges-Pompidou (HEGP – AP-HP) and laboratories shared by Inserm, ESPCI Paris (École Supérieure de Physique et de Chimie Industrielles), Université Paris Sciences et Lettres (PSL) and CNRS (Institut Physique pour la Médecine Paris and Institut Langevin).
“With our innovative NIUT device, we aim to develop and validate for the first time a completely non-invasive approach to aortic valve repair, making it accessible to a greater number of patients, who find themselves without a medical solution today. Our proposal completes the therapeutic arsenal for aortic stenosis“, says Maurice Delplanque, Chief Operating Officer at Cardiawave.
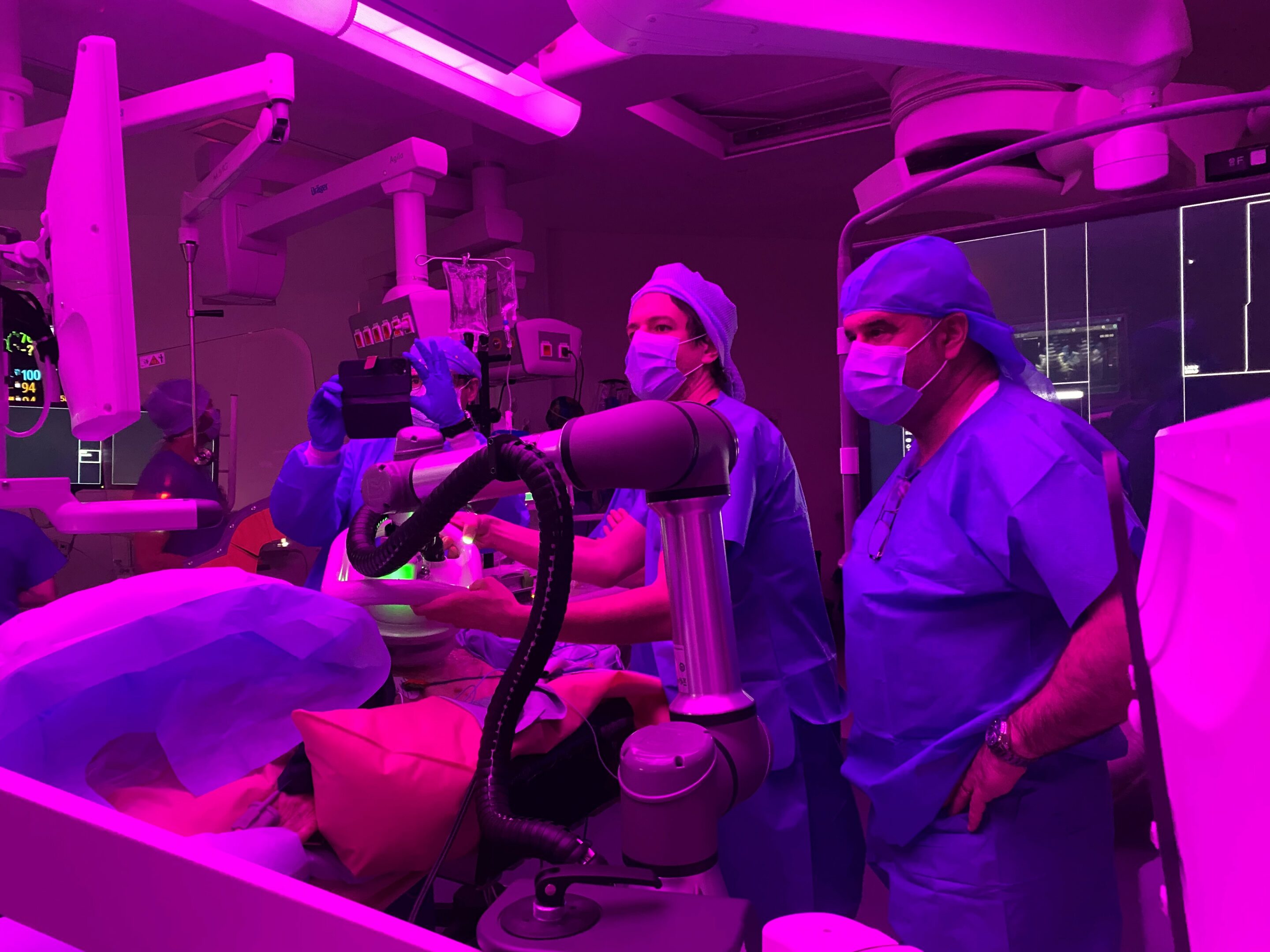
Valvosoft® is a cutting-edge collaborative robotic therapy that accompanies the physician’s movements.
In general, a one-hour session is sufficient. An applicator, equipped with both an ultrasound transducer for therapy and an ultrasound probe to visualize the area being treated, sits at the end of a robotic arm to deliver the therapy with precision. This applicator is placed on the patient’s chest at the level of the heart valve to be treated.
“Ultrasound pulses will focus on the area to be treated and reach pressures that will generate cavitation bubbles,” explains Maurice Delplanque.
“These will implode very rapidly, emitting a wave and creating microcracks in the calcifications present in the valve leaflets. The tissue becomes more supple, the mobility of the leaflets is increased and the passage of blood is facilitated”. “The patient then feels an improvement and can go home“, enthuses Mathieu Pernot, Inserm Research Director in the Physics for Medicine laboratory, who was behind this feat with Professor Emmanuel Messas and physicist Mickaël Tanter, Director of the Physics for Medicine Institute in Paris.
Following animal trials, a First-In-Human feasibility and safety study was launched in 2019: the “Valvosoft® FIM Study”. The results were published in The Lancet on November 13, 2023.
40 patients ineligible for valve replacement were recruited in France (Hôpital Européen Georges-Pompidou, AP-HP, Paris), the Netherlands (Hôpital Amphia, Breda) and Serbia (University Clinical Center of Serbia, Belgrade). The 40 patients were followed up at 1, 3, 6, 12 and 24 months. A significant improvement in cardiac function and quality of life was observed, reflected in particular by a 10% increase in the average opening area of the aortic valve. In the future, it may be possible for doctors to offer patients a new session depending on the evolution of their aortic calcification.
“This represents a real revolution for patients with calcified aortic stenosis. Today, the only solution is valve replacement, which is a very complex operation. The NIUT device developed by Cardiawave is a highly promising non-invasive technology that requires neither anesthesia nor ionizing radiation“, says Mathieu Pernot, Inserm Research Director in the Physics for Medicine Laboratory.
The next phase required to obtain CE marking, authorizing marketing, is a confirmatory pivotal study (“Valvosoft® Pivotal Study”), which began in June 2022. 60 patients were enrolled and treated in 11 hospitals in France, the Netherlands and Germany.
Valvosoft®, the world’s only non-invasive treatment for aortic stenosis, is currently intended exclusively for clinical investigations, pending CE marking. The medical device could be marketed in the near future.