FRENCH HEALTHCARE FOR WOMEN: Two members of the French Healthcare association have recently developed innovations that could change the lives of all patients with endometriosis, or suspected endometriosis, throughout the world. Until now, endometriosis has been considered a mysterious and uncomfortable condition, with inaccurate and sometimes invasive diagnosis and treatment.
Ziwig: a world first for the early diagnosis of endometriosis should transform women’s healthcare internationally
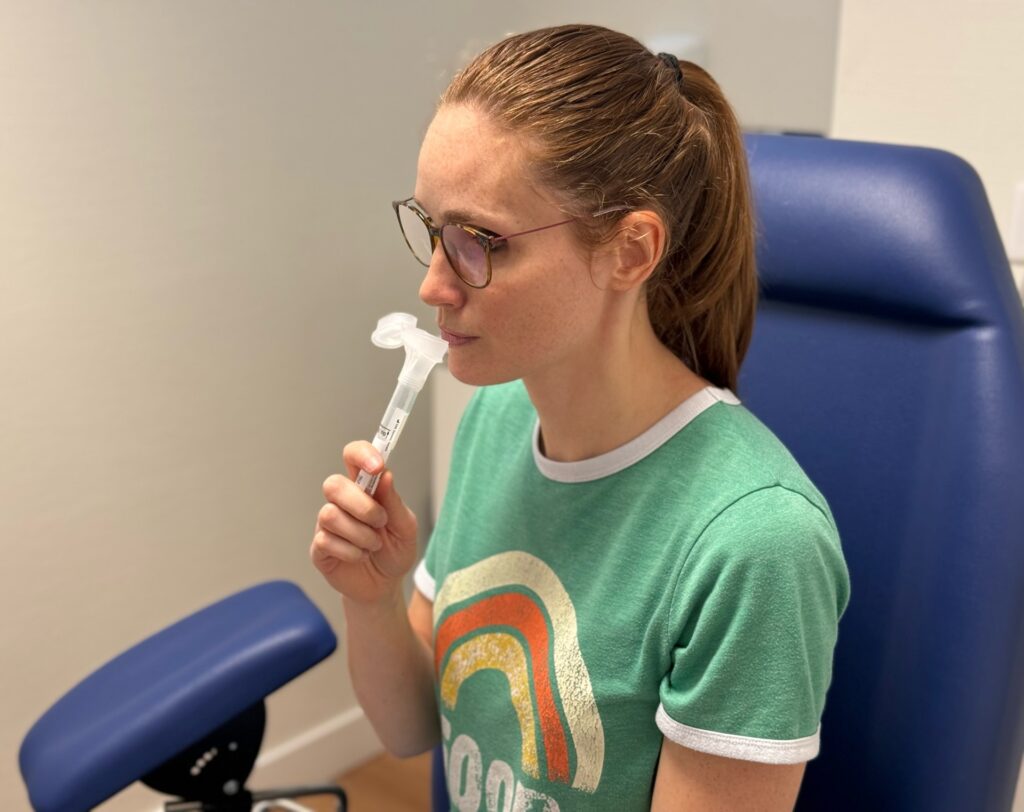
Ziwig, a pioneering biotech in women’s healthcare, is revolutionising the diagnosis of endometriosis with a ground-breaking innovation: the first saliva test capable of accurately detecting the disease.
By combining molecular biology, artificial intelligence and digital health, the company is rapidly establishing itself internationally, demonstrating expertise that is unique in the world.
Norbert Nabet, Director of Public Health, and Yahya El Mir, CEO, take us behind the scenes of this major breakthrough and Ziwig‘s future prospects for improving people’s health over the long term.
Interview with Norbert Nabet, Director of Public Health, and Yahya El Mir, Founder & Chairman, Ziwig
Can you tell us about your innovation for diagnosing endometriosis, and the specific needs it meets?
NN: Endometriosis is a chronic, polygenic disease that potentially affects all women, including those who have gone through menopause or who have already undergone surgical treatment to remove their uterus. The multiple genes involved are the subject of sustained research, but not of perfect knowledge. After numerous observations, we realised that saliva can be used to identify typical micro-arn configurations. We have isolated and analysed these transcriptomic ‘signatures’ and determined that they can diagnose the presence of endometriosis with a 97.3% certainty rate.
The ‘Endotest’ is therefore a major French innovation, enabling all forms of endometriosis to be detected by means of a simple saliva analysis. This non-invasive method offers remarkable accuracy and performance. It represents an unprecedented scientific advance in the world of diagnostics, with potential applications extending to other pathologies, including gynaecological, ovarian cancer and neurological diseases.
What impact has Ziwig had on the knowledge and diagnosis of endometriosis?
NN : In France, the diagnostic pathway for endometriosis is currently based on several stages: identification of suggestive symptoms, followed by ultrasound and MRI, which can only detect lesions on the organs, whereas endometriosis progresses before these lesions appear. In this case, these imaging tools are not enough to establish a definitive negative diagnosis.
In France, the salivary test developed by Ziwig then acts as a diagnostic tool, analysing numerous microRNA sequences present in saliva. These biomarkers can reveal dysfunction at an early stage, enabling a virtually certain diagnosis to be made, whereas imaging only detects established lesions at an advanced stage in the progression of the disease.
Ultimately, we hope that this test will be able to completely replace laparoscopy, which is currently used as a last resort to detect these lesions. This invasive procedure can be counterproductive in terms of patient care, as it carries the risk of complications and worsening of the disease. We are convinced that non-invasive liquid biopsies such as Endotest are alternatives to these surgical procedures and, more generally, simplify the management process and improve patients’ quality of life. In short, a logical and necessary development in the history of women’s health.
These invasive and less effective procedures also explain the average diagnostic delay of around ten years. Another clear advantage of the test is the patient’s independence from the operators, since she can carry out the test herself without any medical expertise, which, given the scarcity of specialist gynaecologists or radiologists in the country, is a real advantage in terms of access to care.
This misdiagnosis also means that the prevalence of the disease is calculated only approximately. Although one woman in 4 suffers from chronic pelvic pain, this does not always mean that endometriosis is present: it is now estimated that the disease affects one woman in ten. On the other hand, a lot of people have heard that the pain is normal, and some women have never thought about the disease, or obtained a diagnosis or treatment.
Our early diagnosis solution could therefore enrich these epidemiological data and change our understanding of the human being: the analysis of micro-arns provides a new level of insight into the workings of human biology. In fact, they were the subject of the Nobel Prize for Medicine awarded in 2024.
American researchers are currently carrying out similar work, but focusing on the inside of damaged cells and how to treat them: this new knowledge of the pathophysiology of the disease opens up the possibility of new ways of intervening at the root of the disease, avoiding hormone therapy or surgery. Endometriosis is often regarded as incurable or as requiring extensive and sometimes traumatic treatment and surgery. Recently, however, new, more reassuring prospects seem to be emerging.
You mentioned your work on other gynaecological and neurological diseases… How are you working to improve the diagnosis of these diseases?
NN : We are already working on advanced versions of our tests, aimed at detecting with greater precision whether endometriosis is superficial, whether it affects the deep organs, or whether it has an impact on fertility.
We are also conducting very advanced studies – soon to be published – to adapt this technology to other pathologies where ease of use and diagnostic accuracy are major challenges. For example, Ziwig could soon enable accurate diagnosis of the following conditions:
- The different forms of endometriosis: deep, superficial, with or without impact on fertility, as well as the likelihood of resistance to the treatments envisaged and the risks of adverse effects.
- Ovarian cancer: this disease has an average 5-year mortality rate of 45%, mainly because it is often diagnosed at stages 3 or 4. However, if detected at the earliest stage, the survival rate is 95%. What’s more, with a recurrence rate of 75%, anticipation is crucial. This study is currently the most advanced in our research programme.
- The ten most common conditions associated with pelvic pain and/or bleeding.
- Certain neurological diseases that are still poorly understood, in partnership with the Institut du Cerveau (Brain Institute), and in particular Charcot’s disease, which remains largely enigmatic. Diagnosis is all the more complex because no intrusive approach is possible when it comes to the brain.
As part of this drive, we are working closely with healthcare professionals to provide them with the technological support they need to care for their patients. To this end, we have developed a secure digital platform that collects and processes data, while optimising the management of flows linked to biological analyses carried out by healthcare establishments.
At the request of healthcare professionals, we are also working on an extension of this platform to improve patient follow-up. This system, designed in agreement with the regional endometriosis networks, will make it possible to offer personalised care pathways based on the medical data collected on each patient.
How have you managed to export your expertise on endometriosis diagnostic? How can Ziwig revolutionise access to women’s healthcare internationally?
NN : Gynaecological care pathways vary, of course, from country to country. As mentioned above, in France, the diagnosis of endometriosis is based primarily on medical imaging. On the other hand, in some countries where imaging is less widespread, our test is used as the first line of defence from the moment of clinical suspicion, facilitating its adoption internationally. To date, our diagnostic innovation has been deployed in 21 countries, including India, Brazil, the Middle East and Australia.
YE : Obtaining regulatory approvals represented a considerable amount of work. Our entry into these markets has been made possible by the CE mark, which is recognised in Europe as well as in certain countries in the Middle East and Asia. We are also well on the way to obtaining FDA approval in the United States. Over and above the regulatory aspects, the accessibility of our technology and its integration into the treatment process are major challenges for patients. Our test is currently the subject of four reimbursement procedures.
In France, it is covered by the ‘innovation package’, allowing early payment by the social security system for 25,000 patients in 80 establishments, pending registration under the general law. A clinical study is being conducted on the first 2,500 cases included in this programme. Its results will enable the CNAM to assess the definitive reimbursement arrangements, and could ultimately lead to a review of gynaecological practices, in particular by reducing systematic recourse to surgery.
In the Middle East, a number of reimbursement schemes have also been set up in response to fertility issues, a major problem in this region. Endometriosis is the leading cause of infertility in women.
The operational deployment of these reimbursement systems, notably in Saudi Arabia and Israel, and very shortly in the United Arab Emirates, is recent and bears witness to a rapidly accelerating global dynamic, driven by Ziwig. These advances are attracting a great deal of international interest, given the persistently high level of diagnostic error and the demand from patients around the world for innovations of this kind. They are suffering from the considerable backwardness of most healthcare systems when it comes to treating women’s pathologies. These developments are taking place against a backdrop of growing societal awareness, a driving force behind promising transformations.
With this in mind, we recently gave a number of interviews to the German media, which were widely echoed: many patients in Germany are now calling for a national roadmap to be put in place, and even for a European Union-wide strategy to be rolled out.
Endotest is currently helping to develop a global reference model. Thanks to this innovation, France is in the spotlight and is playing a leading role in defining international standards for the diagnosis and treatment of endometriosis.
This ambition is fully in line with the vision of the French Healthcare Association, which is working collectively to promote a new excellence in the sector, with France at the forefront. The many successes achieved internationally give this expertise unprecedented influence, enabling it to become a benchmark and, ultimately, significantly improve access to care for patients throughout the world.




Endometriosis: Activa and Nutrilab laboratories promise a significant advance in the treatment of pain
Activa Laboratories have developed an innovative approach to the treatment of endometriosis-related pain, based on extensive research into cellular communication and endocellular nutrition, in partnership with Nutrilab Laboratories.
Endometriosis is a chronic gynaecological condition affecting more than 180 million women worldwide, including around 2 million in France. The pelvic pain it causes is disabling, and the various associated disorders have a considerable impact on patients’ quality of life.
In response to these challenges, a partnership between Activa laboratories and Nutrilab has led to the development of an innovative approach based on advances in cellular communication, endocellular nutrition and microgranule technology in nutritional solutions.
Current approaches to managing endometriosis are mainly aimed at relieving pain in the short term with hormonal treatments or painkillers, sometimes combined with surgical management. However, new advances in clinical research are paving the way for more targeted and sustainable solutions.
More precise targeting of pain thanks to sustained clinical research
A randomised, double-blind clinical trial published in leading scientific journals recently demonstrated a significant reduction in the pain associated with endometriosis. In four months, 91.3% of patients using this approach experienced relief, with a 66.1% reduction in pain intensity.
This new approach not only temporarily masks the pain, but also acts on the biological mechanisms involved in the progression of the disease. In particular, by targeting aquaporins, essential molecular transport proteins, it contributes to more effective regulation of inflammatory and hormonal processes.
What’s more, this solution can be easily integrated into the overall management of patients suffering from endometriosis, adapting to the different phases of the female cycle, favouring gradual release for an optimised therapeutic effect, and being compatible with conventional treatments and even medically assisted procreation.
Thanks to the complementary expertise of Activa and Nutrilab, this innovation represents a significant advance in the treatment of endometriosis. The integration of these new approaches into clinical practice could mark a key step towards improving the well-being of women suffering from this condition.